Myoglobin Vs Hemoglobin a Quick Difference and Comparison Note
Myoglobin Vs Hemoglobin, The very basic difference between these terms is that myoglobin a monomeric protein that binds oxygen and supplies it to skeletal muscles, whereas hemoglobin a heterotetrameric protein found in erythrocytes. Hemoglobin transports oxygen from the atmosphere to the body.
There are close kinship relationships between hemoglobin and myoglobin. Both conjugated proteins and their prosthetic group (non-protein part) is the heme group.
also read: Apoptosis and necrosis differences

Let’s compare myoglobin vs hemoglobin side by side, but before we get started I wanna say You watch the video at the end of this article for a better understanding.
Myoglobin vs Hemoglobin Differences
| Myoglobin | Hemoglobin |
| 1. Myoglobin is a protein of the heart and all skeletal muscles. | Hemoglobin is a protein of red blood cells. |
| 2. It consists of single polypeptide chain. | It is a quaternary structure protein consists of two pairs of alpha subunits and beta. |
| 3. Myoglobin is a monomer. | Hemoglobin is a tetramer. |
| 4. It has hyperbolic adsorption. | It gives a marked sigmoid. |
| 5. Its affinity for oxygen is greater. | Its affinity for oxygen is less. |
| 6. The molecular weight is about 17,000. | The molecular weight is 64000. |
| 7. It contains 153 amino acid residues. | It contains 287 amino acid residues. |
| 8. Moreover, the concentration in blood is low in RBC. | The concentration in blood is high in RBC. |
| 9. It present in muscles cells. | It is present all over the body. |
| 10. Also known as Mb. | Also known as Hb. |
| 11. It has the function of storing oxygen in muscle tissue. | The main function is the transport of oxygen. |
However, there are a lot of differences between myoglobin and hemoglobin but there are also similarities between them.
Now let’s see a bit of similarity between these two terms.
Myoglobin vs hemoglobin Similarities
- Both are two globular proteins with an iron nucleus.
- They are colored. Its color depends on the oxidation state of the iron they contain and the partial pressure of O2.
- Both transport oxygen and therefore they have structural similarities.
- They bind molecules like CO or NO.
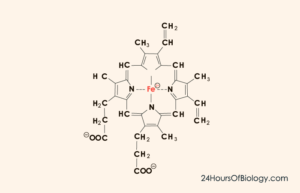
- They contain heme groups form by 4 pyrrole rings that complex a divalent iron ion Fe + 2.
Myoglobin definition and structure
Myoglobin is a complex protein of the third level of structural organization. It is a single-chain protein found in muscle tissue where it serves as an intracellular storage site for oxygen. It is able to bind oxygen to muscle cells, which gives them the energy to contract.
Myoglobin is a relatively small protein that contains a heme group with an iron atom, and whose function is to store and transport oxygen.
Structure:
Myoglobin is a globular protein consisting of a single chain of approximately one hundred and fifty amino acids. Additionally, its molecular weight is approximately 18 Kd. Excluding the two His residues involved in the oxygen fixation process, the interior of myoglobin contains only non-polar residues.
Moreover, it has a protein called heme, which consists of iron and responsible for the red and brown color of protein.
is myoglobin globular? Exactly, myoglobin is a globular protein category of singular chain structure possessing a prosthetic heme group at the pivot. Further, there is an iron attached at the pivot of the heme group associates with Hexa ligands, tetra nitrogenous atoms of the porphyrin ring, an O2 molecule, and an imidazole side chain remnant His-64.
Hemoglobin definition and structure
Hemoglobin is the most important element in the blood, an iron-containing protein. It is the key part of red blood cells. The main functions of hemoglobin are oxygen transfer and buffer function. In addition, hemoglobin is able to bind a small amount of carbon dioxide in the tissue and release it in the lungs.
However, both a deficiency and an excess of this protein are dangerous.
Normally, the hemoglobin content in the blood of men is slightly higher than that of women. Its level is variable. It changes depending on age, habits, and lifestyle, health status.
Structure:
Hemoglobin has many structural similarities with the myoglobin and is capable of binding molecular oxygen in a reversible manner. But, while, myoglobin is confined to the muscles and peripheral tissues in general, hemoglobin is found in erythrocytes or red blood cells.
Hemoglobin is a protein with a quaternary structure, that is, it is made up of four polypeptide chains: alpha, beta, gamma, and delta. The molecular weight is about 66.8 kDa. α subunit contains 141 amino acid residues, the β subunit contains 146 amino acid residues. It can carry up to four oxygen molecules.
Because each chain has a heme protein group, there are 4 iron atoms in each hemoglobin molecule.
Hemoglobin:
Mature hemoglobin is a heterotetrameric [α(2):β(2)] hemeprotein discovered in erythrocytes where it’s capable of bonding with oxygen in carrying bounded oxygen everywhere the body and lungs, where it’s utilized in aerobic type of metabolic pathways.
Every hemoglobin tetramer subunit possesses a prosthetic group heme identical to that specified for myoglobin. The quaternary hemoglobin composition leads to physiologically significant allosteric synergies within the subunits, a characteristic without monomeric myoglobin, which is contrarily very related to hemoglobin α-subunit.
Hemoglobin Importance:
- Assists in sustaining pH.
- It performs a part in erythrocyte metabolism.
- They serve as physiological operating catabolites.
- It provides blood color.
- Haemoglobin works as a messenger for stocking CO2 as well as O2.
Hemoglobin Types:
- Embryonic hemoglobin
- Glycosylated hemoglobin
- A1 (Hb-A1) hemoglobin
- A2 (Hb-A2) hemoglobin
- A3 (Hb-A3) hemoglobin
Myoglobin:
The tertiary composition of myoglobin is that of a conventional water solvable globular protein. Its secondary composition is rare in which it holds a really large portion (75%) of the α-helical secondary composition.
All myoglobin unit holds a sole heme crowd embedded into protein hydrophobic cleft.
Hydrophobic cooperations within the hydrophobic amino acid R groups on the inside of the cleft in the protein & tetrapyrrole ring has fully maintained the conjugated heme–protein. In extension, a nitrogen atom/particle from a histidine R group placed over the sphere of the heme ring is correlated by an iron atom moreover maintaining the cooperation within the heme & protein.
In oxymyoglobin, the leftover bonding place on iron atom (6th bonding site) is O2 owned, whose bonding is maintained by 2nd histidine remnants.
Myoglobin importance:
- Aid in controlling body heat.
- Bring O2 to the muscle tissues.
- It supports the body at the starved condition of O2, particularly in anaerobic conditions.
- Myoglobin possesses a great empathy for O2 binding, which ables it to stock it in muscles effectually.
Key Differences Myoglobin Vs Hemoglobin.
- Hemoglobin possesses 4 chains of 2 distinctive categories. as alpha, gamma, beta, and epsilon which depend on hemoglobin variety, & compose a tetramer construction, while myoglobin includes single polypeptide series so-called monomer. both possess the intermediate ion as iron & ligand of O2 binding.
- Hemoglobin is carried onward with the blood to all body parts also assists in conveying O2; Myoglobin serves O2 solely to muscles does help while there is a lot of O2 demand by blood.
- Hemoglobin ties by O2, CO2, CO, NO, BPH including H+, on the other side myoglobin ties solely with O2.
- Hemoglobin also recognized as Hb available in greater quantity in Red Blood cells while myoglobin is likewise recognized as Mb.
- It provides hemoglobin ahead systematically with blood to the whole body while myoglobin provides O2 solely to muscles.
Moreover, both molecules possess O2 binding capacity, as presented before.
Conclusion (Myoglobin Vs Hemoglobin):
hemoglobin & myoglobin are fairly & physiologically necessary, because of their oxygen binding strength. These were the primary 3D molecules discovered through X-ray crystallography. Anomalies in the components may begin to critical disorders.
Hemoglobin and myoglobin oppose in the requiredO2 affinities. But their core metal ion is identical, onward with identical ligand required particles/molecules. Both are essential to the body, without them we can’t imagine the life.
You may also enjoy reading: