
What is the difference between diffusion and osmosis
The osmosis and diffusion difference is that diffusion is the movement of a liquid substance from higher to the lower area of concentration while osmosis is the movement from lower to the higher area of concentration through a membrane.
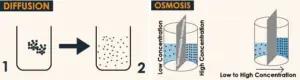
A membrane is a barrier that may encapsulate the different molecules and allow only selective molecules to pass through it. A membrane can be between two different molecule streams and allows some selective molecules from one stream to pass to another stream. We can also consider the membrane as a wall between two different natural environments.
Diffusion and osmosis difference
| Diffusion | Osmosis |
| 1. Movement always works down the concentration gradient. | 1. Movement always works up the concentration gradient. |
| 2. It does not Requires membrane. | 2. Requires membrane. |
| 3. Always particles move from high to low. | 3. Always particles move from low to high. |
| 4. molecules are free to move. | 4. Molecules are not free to move, only selective molecules are allowed to move. |
| 5. It can take place in any medium. | 5. It only takes place in a medium having a semi-permeable membrane. |
| 6. It can be applied to liquids, solids, and gases. | 6. Only applicable to liquids. |
| 7. It is a reversible process. | 7. It is an irreversible process. |
| 8. Insist on the system towards stability (equilibrium). | 8. Do not insist on the system towards stability (non-equilibrium). |
| 9. Spontaneous process. | 9. The spontaneous process since the solute is independent of the concentration gradient. |
Examples of osmosis and diffusion
Example of osmosis:
Suppose You are fasting for a day and its too hot weather and You do not have to drink water in order to satisfy Your thirst because You are fasting. You go to a freshwater pool and dip Your legs inside the water.
What will happen?
After sometimes You start feeling that Your thirst or Your desire to drink water will gradually go down. All this happens because there is a higher concentration of water in the pool than Your body so it starts moving inside Your body through pores on Your body. As we already know that in osmosis liquid substance moves from its higher to the lower area of concentration.
There are dozens of real-life biological examples of osmosis. soon will be updated.
Osmosis Types
There are three types of osmosis Hypertonic, Isotonic, and Hypotonic osmosis.
Hypertonic: In hypertonic osmosis water moves from outside of the cell to inside of the cell because of higher solute concentration in the outer environment
Isotonic: In isotonic osmosis water moves inside the cell from outside and at the same time exits from the cell to outside so there is no water movement.
Hypotonic: In hypotonic osmosis water keeps going inside the cell until an equilibrium state is achieved.
Example of diffusion:
Suppose a person probably Your friend is standing in a corner of a room holding perfume in his/her hand and You at the other corner of the room standing. Your friend let’s suppose presses the perfume and after some seconds it reaches to You and You experience the pleasant perfume smell.
How it reaches You? and the answer is simply by diffusion
As we already know that in diffusion particles move from their higher concentration area to the lower concentration area. Simple is that.
Types of diffusion:
There are mainly two types of diffusion passive diffusion and facilitative diffusion.
Passive Diffusion: Movement of molecules from higher to the lower area of concentration until the concentration gets equal across the medium.
Facilitative Diffusion: Spontaneous movement of molecules across a biological membrane but via transmembrane integral proteins.