Glycogen vs Starch, Knowledge Booster Differences and Comparisons
Glycogen vs Starch, both are carbohydrate forms. Both are considered as sugar reserves in plants as well as in animals. Both contrast in their glycosidic linkages & their tasks as well. Commence from the cellulose which is the monomer of beta glucose and is found in the cell wall of plants. In spite of the fact that their chains have slight contrasts at the branch points.
Glycogen is a multibranched polysaccharide of glucose that fills in as a type of vitality stockpiling in animals (including humans), parasites, and bacteria. The polysaccharide structure speaks to the principle stockpiling type of glucose in the body.
Another name of starch is amylum. Starch is a sugar created by every single green plant that has countless glucose units used to store vitality. Plants, for example, potatoes, wheat, corn, rice, and cassava, are on the whole rich with this kind of sugar which is vital for us.*
In the case of humans, Our bodies need vitality to prop us up. On the off chance that we need vitality, we feel frail and our organs can’t work appropriately. Without it, we can’t even move and do even the most essential things like walk or eat. To stay aware of our body’s vitality need, we need to get a generous admission of sugar or glucose which is a vitality ingredient for our cells.
Difference comparison of starch vs glycogen.
| Glycogen | Starch | |
| 1. | It exists in animals and in plant that don’t possess chlorophyll. | It is found in all plants |
| 2. | Glycogen is likewise homopolysaccharide & found in creatures as their sugar reservoir; it is additionally found in growths and plants that don’t contain chlorophyll. | Starch is additionally the homopolysaccharides & as the sugar reservoir plants and the dietary hotspot for the creatures. |
| 3. | Glycogen possesses α(1-6) & α(1-4) glycosidic linkages within their monomers at branch points. | It possesses glucose deposits as α(1-4) glycosidic bonds in amylose, while in amylopectin α(1-6) glycosidic linkages at spreading focuses, in any case, α(1-4) linkages. |
| 4. | Glycogen possess chains that are short and profoundly stretched. | They are curled and unbranched (amylose) or since quite a while ago, spread (amylopectin) |
| 5. | It makes small granules. | It makes grains. |
| 6. | The solvent in the little degree, as they are profoundly expanded. | Amylose is soluble in water, and amylopectin is water-insoluble. |
| 7. | Glycogen is additionally homopolysaccharide and found in creatures as their starch reservoir, it is likewise found in organisms and plants that don’t contain chlorophyll. | Starch is additionally the homopolysaccharides and as the sugar hold of the plants and the dietary hotspot for the creatures. |
Glycogen vs Starch in detail.
Glycogen Definition.
Glycogen, also named as starch of animals, however, found in plants that don’t contain chlorophyll like fungi & yeast. It is additionally the homopolysaccharide having the glycogen bonds or linkages like that of the amylopectin, with the more branches. Glycogen has the α(1-4) glycosidic bonds with the α(1-6) glycosidic bonds at the branch points (happening at each 8 to 12 deposits).
Glycogen employes as one of two types of vitality hold, glycogen is considered being triglyceride stores in fat tissue (i.e., muscle to fat ratio) for long haul stockpiling. In people, glycogen is made and put away fundamentally in the cells of the liver and skeletal muscle.
In the liver, glycogen can make up 5–6% of the organ’s new weight, and the liver of a grown-up weighing 1.5 kg can store around 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low engrossment(1–2% of the bulk), and the skeletal muscle of a grown-up gauging 70 kg stores approximately 400 grams of glycogen.
The measure of glycogen put away in the body—especially inside the muscles and liver, generally relies upon physical preparation, basal metabolic rate, and dietary patterns. Modest quantities of glycogen are additionally found in different tissues & cells, including the kidneys, red blood cells, white blood cells, and glial cells in the brain. The uterus likewise stores glycogen during pregnancy to sustain the incipient organism.
Roughly 4 grams of glucose are available in the blood of people all time; in abstained people, blood glucose is kept up consistent at this level to the detriment of glycogen stores in the liver and skeletal muscle.
Glycogen stores in skeletal muscle fill in as a type of vitality stockpiling for the muscle itself; be that as it may, the breakdown of muscle glycogen blocks muscle glucose take-up from the blood, accordingly expanding the measure of blood glucose accessible for use in other tissues. Liver glycogen stores fill in as a store of glucose for use all through the body, especially the focal apprehensive system. The human mind expends around 60% of blood glucose in abstained, inactive people.
Glycogen is simple of starch, a glucose polymer that works as vitality stockpiling in plants. It has a structure like an amylopectin (a part of starch), yet is more widely branched & conservative than starch. Both are white powders in their dry state. Glycogen is found as granules in the cytosol/cytoplasm in numerous cell types and assumes a significant job in the glucose cycle. Glycogen shapes a vitality hold that can be immediately assembled to meet an unexpected requirement for glucose, yet one that is less reduced than the vitality stores of triglycerides (lipids). As such it is likewise found as vitality save in numerous parasitic protozoa.
Glycogen Structure.
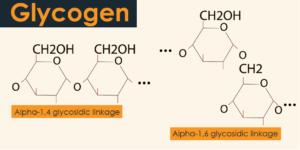
Glycogen structure is a spread biopolymer comprising of straight chains of glucose buildups with a normal chain length of around 8–12 glucose units.
Glucose units are connected together straightly by α(1-4) glycosidic bonds starting with one glucose then onto the next. Branches are connected to the chains from which they are fanning out by α(1-6) glycosidic bonds between the primary glucose of the new branch and glucose on the stem chain.
Glycogen in muscle, liver, and fat cells is put away in a hydrated structure, made out of three or four parts of water for each glycogen part related to 0.45 millimoles (18 mg) of potassium per gram of glycogen.
Glucose is an osmotic particle, and can effectively affect osmotic weight in high focuses potentially prompting cell harm or passing whenever put away in the phone without being modified. Glycogen is a non-osmotic atom, so it tends to be utilized as an answer for putting away glucose in the cell without disturbing osmotic pressure.
Starch Definition.
Starch or amylum can be defined as a polymeric sugar comprising of various glucose units joined by glycosidic bonds. This polysaccharide is created by most green plants as vitality stockpiling. It is the most well-known sugar in human weight control plans and is contained in huge sums in staple nourishments like potatoes, maize (corn), rice, and cassava, just as in the grain Emmer wheat (Triticum amyleum), from which is created a developed white starch.
Unadulterated starch (pure/original) is a tasteless white & unscented powder that is insoluble in chilled water or liquor. It comprises of two kinds of particles: the helical & linear amylose & the stretched amylopectin.
Contingent upon the plant, starch normally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the glucose store of animals, is an all the more exceptionally spread adaptation of amylopectin.
In industry, starch is changed over into sugars, for instance by malting, and matured to deliver ethanol in the production of lager, whisky, & biofuel. It is handled to deliver a considerable lot of the sugars utilized in prepared nourishments. Blending most starches in warm water delivers a glue, for example, wheatpaste, which can be utilized as a thickening, solidifying or sticking purposes.
The greatest modern non-food utilization of starch is as a cement in the papermaking procedure. Starch can be applied to parts of certain pieces of clothing before pressing, to solidify them.
Starch Structure.
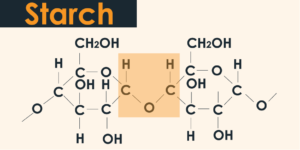
While amylose was believed to be totally unbranched, it is currently realized that a portion of its atoms contains a couple of branch points. Amylose is a lot littler particle than amylopectin. Around one-fourth of the mass of starch granules in plants comprise of amylose, despite the fact that there are around multiple times more amylose than amylopectin particles.
Starch particles orchestrate themselves in the plant in semi-glasslike granules. Each plant species has a one of a kind starch granular size: rice starch is moderately little (around 2 μm) while potato starches have bigger granules (up to 100 μm).
Starch gets solvent in the water when warmed. The granules swell and burst, the semi-glasslike structure is lost and the littler amylose atoms begin draining out of the granule, framing a system that holds water and expanding the blend’s thickness. This procedure is called starch gelatinization.
During cooking, the starch turns into the glue and increments further inconsistency. During cooling or delayed stockpiling of the glue, the semi-translucent structure mostly recuperates and the starch glue thickens, ousting water. This is for the most part brought about by retrogradation of the amylose. This procedure is answerable for the solidifying of bread or staling, and for the water layer on the head of a starch gel (syneresis).
Some developed plant categories have unadulterated amylopectin starch without amylose, known as waxy starches. The most utilized is waxy maize, others are glutinous rice and waxy potato starch. Waxy starches have less retrogradation, bringing about a more steady glue. High amylose starch, amylomaize, is developed for the utilization of its gel quality and for use as a safe starch (a starch that opposes absorption) in food stuff.
Manufactured amylose produced using cellulose has an all-around controlled level of polymerization. Along these lines, it very well may be utilized as a potential medication convey carrier.
Certain starches, when blended in with water, will deliver a non-newtonian liquid once in a while nicknamed “oobleck”.
What is the Structural Differences Starch vs Glycogen?
- Mass: Starch is littler in mass contrasted with glycogen: This is on the grounds that glycogen has a more extended chain contrasted with starch which makes the atoms that interface it has a bigger mass when the molar mass is determined. Starch is a shorter polysaccharide, consequently, having a littler molar mass contrasted with glycogen.
- Creation: Starch is a polymeric sugar comprised of glucose consolidated: The glucose segments of starch are delivered during photosynthesis where plants utilize light, water, and carbon dioxide. Glycogen is created from glucose where glucose is changed over to glycogen when the insulin levels are high which demonstrates that the body has overabundance glucose and along these lines, it is put away. In contrast to starch, glycogen is created by the change of glucose in creature tissues.
- Reason: The primary purpose behind glycogen in the body is to keep up steady degrees of glucose in the blood which gives vitality in the tissues: This is encouraged by the degree of insulin in the body. Elevated levels of insulin demonstrate low degrees of glucose and hence the glycogen polymer is changed over into glucose because of glucagon. Starch in plants is put away because of the creation of overabundance sugars. In creatures, starch is separated into monomer segments known as glucose and afterward changed over into complex polymers known as glycogen.
- Manufacturing: Starch is manufactured and found in every green plant: Starch is principally shaped in green plants as vapid granules of round shapes. The essential crude materials that are required for the development of starch are daylight, water, and carbon dioxide. Glycogen, on the other hand, is shaped inside the animal’s tissues and is then put away in the liver. The arrangement of glycogen is reliant fair and square of insulin in the body. Higher insulin levels mean higher glycogen levels.
- Particles: The most straightforward type of starch is amylose: The polysaccharide is shaped by glucose joined by glycosidic bonds making the accompanying atomic equation (C_6 H_10 O_5)_n H_2 O. It comprises of two sorts of particles, the helical & linear amylose and branched amylopectin. The atomic equation of glycogen is C_24 H_42O_21 which speaks to its structure. It is made out of numerous glucose iotas which are associated by bonds to make up the total structure of glycogen.
- Existence: Starch is available in green plants while glycogen is available in animals: Starch is found in plants as put away complex polymer made out of interconnected glucose molecules. It is for the most part put away in the seeds, foods grown from the ground of its follows found in the leaves. With creatures, glycogen is put away for the most part in the liver, skeletal muscles and in the blood. The nearness of glycogen in creatures guarantees a consistent progression of vitality to encourage the ordinary working of the body.
Video eplaination.
Conclusion:
Glycogen and Starch are two fundamental wellsprings of glucose that give the human body the vitality required so as to perform everyday undertakings. These two glucose wellsprings are then changed over into starches by the body and circulated to each and every phone for some time in the future. Glycogen also known with the name animal starch, is a wellspring of vitality that can be found in creatures as it were. Glycogen comprises of a solitary particle and its structure is stretched absolutely. The animal’s liver & muscles are dependable in the formation of glycogens. The glycogens go about as a crisis hold when the human body unexpectedly needs a plentiful measure of vitality, for instance, in crisis circumstances like fire and flood. Starch, the equivalent with glycogen, is another wellspring of vitality that can be found in plants as it were. Starch can for the most part be found in staple nourishments.
You may also enjoy reading: